Cesium Periodic Table Protons And Neutrons
Removes air traces in vacuum tubes. The sum of protons and neutrons are called nucleons because these are located in the nucleus of the atom.

Pin On Math And Science Videos
Cesium Cs 137 is a radioactive isotope of cesium with an atomic mass of 139 and potential application in radiotherapy.

Cesium periodic table protons and neutrons. The number of protons is equal to the number of electrons unless theres an ion superscript listed after the element. Chemical state Binding energy Cs3d 52. 55 electrons green bind to the nucleus with a single relatively unstable electron in the outer shell ring.
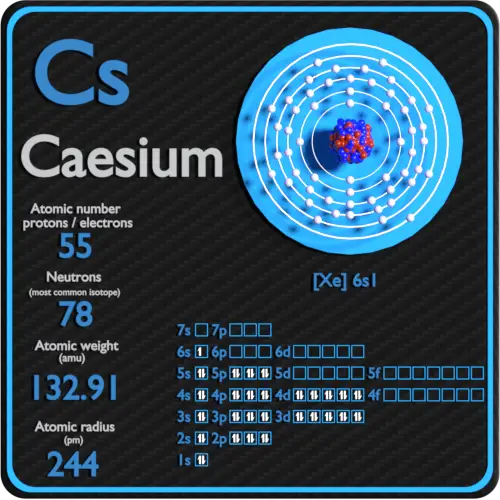
Cesium is a soft ductile metal. 55 Number of Neutrons. 1329054519 atomic mass units.
Table of Elements Cesium Cesium X-ray photoelectron spectra cesium electron configuration and other elemental information. The positively charged subatomic particle is called proton. Element that is the only nonmetal on the left side of the Periodic table protons and neutrons only 2 particles to determine mass of the element.
Neutron is a subatomic particle that is present in nucleus with no charge. NA Binding energies of common chemical states. It is a soft silvery-golden alkali metal with a melting point of 285 C 833 F which makes it one of only five elemental metals that are liquid at or near room temperature.
Caesium IUPAC spelling also spelled cesium in American English is a chemical element with the symbol Cs and atomic number 55. 1873 gcm 3 Color. Cesium hydroxide the strongest base known attacks glass.
Cesium reacts explosively with cold water and reacts with ice at temperatures above -116C. Interpretation of XPS spectra. Alkali Metal Crystal Structure.
13290546 amu Melting Point. The easiest way to find the number of protons neutrons and electrons for an element is to look at the elements atomic number on the periodic table. 1837 grams per cubic centimeter.
Only one stable isotope of cesium occurs naturally cesium-133. That number is equal to the number of protons. The nucleus consists of 55 protons red and 78 neutrons blue.
An atom of cesium eqrm Cs eq has 55 protons 55 electrons and 77 neutrons. 6784 C 95155005 K 125312 F Number of ProtonsElectrons. 285 C 30165 K 833 F Boiling Point.
Cesium Cs 137 is prevalent due to its spontaneous production which occurs as a result of nuclear fission of other radioactive materials such as uranium and plutoniumThis radionuclide has a relatively long half-life 30 years and decays by emitting beta particles. Cubic Density 293 K. Some radioisotopes are produced in nature by slow neutron.
Cesium Alkali Metal. Francium is the most electronegative element according to the Pauling scale. According to the Allen scale of electronegativity cesium is the most electronegative element.
This most alkaline of metals reacts explosively when it comes in contact with cold water. Cesium is one of the elements in the periodic table and has more than 40 isotopes. 55 the most common isotope of this element.
There are 11 major radioactive isotopes of cesium. It is readily drawn into fine wires. Protons electrons and neutrons are the three subatomic particles that are present in the atom.
Because of it has great affinity for oxygen the metal is used as a getter in electron tubes. Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons. Caesium has physical and chemical properties similar to those of rubidium and potassium.
It is also used in photoelectric cells as well as a catalyst in the. Diagram of the nuclear composition and electron configuration of an atom of caesium-133 atomic number. Cesium gallium and mercury are the only three metals that are liquid at room temperature.
Lithium is located in Group 1 of the Periodic Table which element has similar characteristics of Lithium. Cesium melts at the relatively low temperature of 28 o C 82 F so like mercury it is liquid at moderate temperatures. 724 eV Experimental information.
Electrons revolve around the nucleus in fixed orbital path. Numerous artificial radioactive isotopes have been produced. 112 Zeilen Vanadium has 23 protons 28 neutrons and 23 electrons.
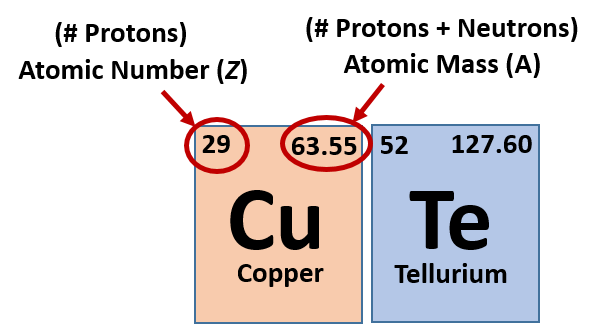
To find the number of neutrons. The ______________ is the number of protons neutrons in the nucleus of an atom. Make up the nucleus center of atom.

Calculating Parts Of An Atom Practice Worksheet Bundle Practices Worksheets Chemistry Worksheets Worksheets

Thmg144 The Periodic Table Part Ii The Haz Mat Guys
Caesium Atomic Structure Stock Image C013 1605 Science Photo Library
The Periodic Table By Energy Levels

Properties Of Atoms And The Periodic Table Chapter Ppt Download

Class 11th Chemistry Structure Of Atom 11th Chemistry Chemistry Atomic Structure

Ppt How To Find The Number Of Protons Neutrons Electrons For An Element On The Periodic Table Powerpoint Presentation Id 3063791
How Many Times Smaller Than An Atom Are Protons Neutrons And Electrons Quora

Periodic Table Flashchards Element Properties Newton Desk Earth Science Lessons Periodic Table Earth Science Projects

F2016 Bis2a Lecture03 Reading Igo

Learning The Periodic Table Of Elements What Are Atoms Atoms Are The Simplest And Smallest Particle Composed Of Protons Electrons And Neutrons The Ppt Download
Caesium Periodic Table And Atomic Properties

18 Ar Argon Electron Shell Structure Schoolmykids Atomic Structure Periodic Table Of The Elements Electron Configuration
Ch104 Chapter 2 Atoms And The Periodic Table Chemistry

Family Of Alkali Metals Alkaline Earth Metals Alkaline Earth Metals Noble Gas Alkali Metal

Calculating Parts Of An Atom Practice Worksheet 4 Practices Worksheets Chemistry Worksheets Worksheets

Periodic Table Poster Printable Science Wall Art Classroom Etsy Periodic Table Poster Posters Printable Educational Wall Art

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Electrons


Posting Komentar untuk "Cesium Periodic Table Protons And Neutrons"